Digital Health & DiGA
We guide you through the DiGA fast-track procedure
The framework conditions for market access of digital health applications (DiGA) and other digital healthcare innovations have never been better. If you have developed a digital solution that demonstrably contributes to improving healthcare in Germany, all lights are green for you. The inav offers you all services under one roof to pave the way for your digital innovation to enter the market.
With admission to the DiGA directory of the BfArM, digital health applications such as apps, web applications and other digital medical devices become eligible for reimbursement under the Statutory Health Insurance system. This means the application can be prescribed by physicians or psychotherapists on prescription, depending on the indication.
In order to be listed in the DiGA directory, each DiGA has to undergo a multi-stage review process in which, amongst other things, proof of evidence is required.
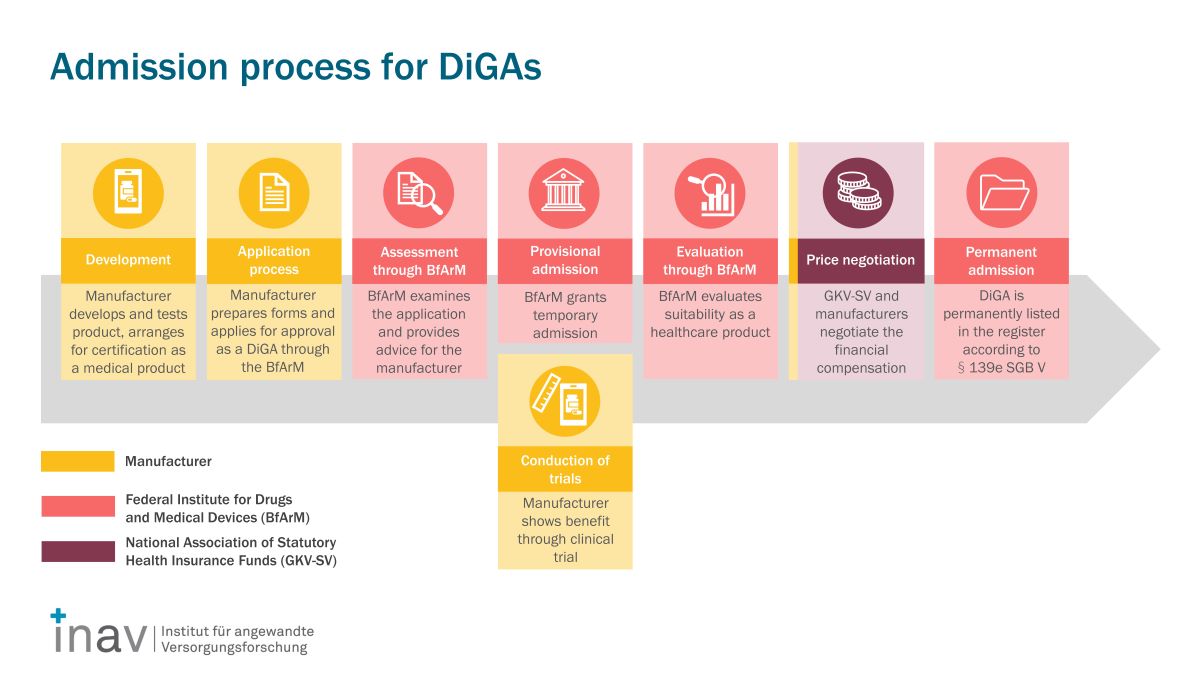
If your goal is to have your digital solution listed in the German DiGA directory, we will support you through the entire fast-track process. This includes strategy consulting, developing the evaluation concept, accompanying appointments with the responsible authority institution (BfArM), conducting the study, developing pricing models, training for price negotiations, and market roll-out in the market.
Experience has shown that there is a particular focus on the preparation of the documents that must be submitted for provisional inclusion in the DiGA directory. In addition to the evaluation concept, this includes a systematic data analysis, the statistical analysis plan (SAP), a systematic literature search, and a study protocol.
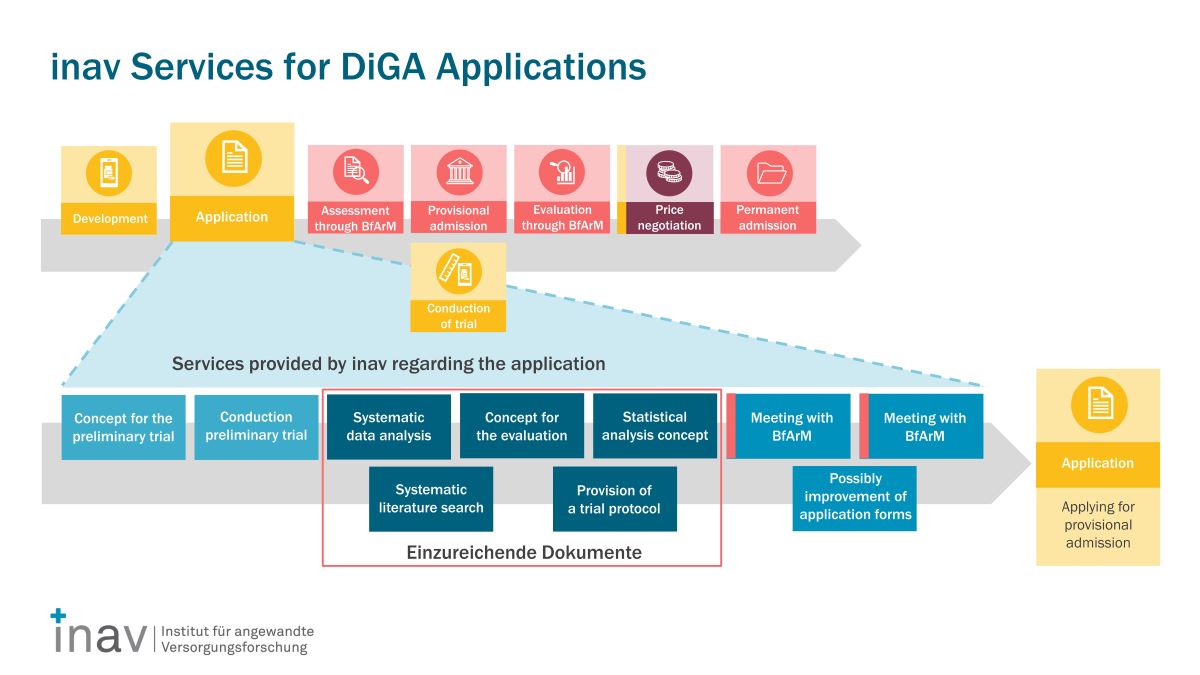
Our module-based service packages can be customized to meet your specific needs and objectives.
What is a DiGA?
The term DiGA stands for Digital Health Application. DiGA could be apps, web applications, or other digital medical devices.
A primary requirement is that the application be certified as a risk class I or IIa medical device (MDR or MDD, respectively). In addition, the application must meet the following criteria:
- The main function of the DiGA is based on digital technologies.
- The medical purpose is achieved through the main digital functions.
- The application supports the recognition, monitoring, treatment or alleviation of diseases or the recognition, treatment, alleviation or compensation of injuries or disabilities.
- The application is used by the patient alone or by patient and healthcare provider together.
Further, the DiGA must be proven to have a positive effect on healthcare, the evidence for this being provided as part of a comparative study.
Accordingly, applications with the following characteristics will NOT be approved as DiGA:
- Applications with the main or sole purpose of primary prevention.
- Applications designed exclusively to enable the reading or controlling of a device
- Applications that are only used by service providers (without the direct involvement of patients)
We examine whether your digital health application meets the general requirements for listing in the DiGA directory and guide you through all further steps in the admission process.
What is the DiGA fast-track procedure?
The DiGA fast-track process is the admission procedure for digital health applications. It is based at the German Federal Institute for Drugs and Medical Devices (BfArM) and is intended to enable rapid access for digital health applications to the healthcare market.
The fast-track procedure offers two different routes leading to a listing in the DiGA directory:
- Final listing in the DiGA directory: A final listing can only be applied for if the manufacturer for the DiGA can already present a comparative study demonstrating a positive healthcare effect, which fulfills the requirements according to Sections 10 to 12 of the Digital Health Applications Ordinance (Digitale-Gesundheitsanwendungen-Verordnung, DiGAV). The study must be fully completed at the time of application.
- Provisional inclusion in the DiGA directory: If there is not yet a study demonstrating positive health care effects, this evidence can be provided as part of a trial phase. The trial phase lasts a maximum of twelve months. Also for the application for provisional admission to the DiGA directory, the application must already meet all requirements according to Sections 3 to 6 of the DiGAV (security, functional capability, quality, data protection and information security) at the time of application. In addition, various documents must be submitted with the application which plausibly demonstrate that the DiGA will contribute to improving health care and that corresponding evidence can be provided within the trial phase. For this purpose, the manufacturer has to attach an evaluation concept, a systematic data evaluation, a systematic literature research, a statistical analysis plan and a study protocol to the application.
inav supports DiGA manufacturers in deciding on the appropriate application path and with all the steps involved in getting listed in the DiGA directory.
What are positive healthcare effects?
According to the Digital Healthcare Act (DVG), positive healthcare effects (positive Versorgungseffekte, pVE) are either a medical benefit (medizinischer Nutzen, mN) or a patient relevant improvement of structure and processes (patientenrelevante Struktur- und Verfahrensverbesserungen, pSVV) in healthcare.
A medical benefit is achieved if one of the following is achieved with the help of the DiGA:
- the improvement of the state of health
- the reduction of the duration of a disease
- the prolongation of survival
- an improvement in the quality of life
Patient relevant structural and process improvements include improvements in the following areas:
- coordination of treatment procedures
- alignment of treatment with guidelines and recognised standards
- adherence
- facilitating access to care
- patient safety
- health literacy
- patient autonomy
- coping with illness-related difficulties in everyday life
- reduction of therapy-related efforts and strains for patients and their relatives
With our many years of experience in the field of evaluation, we can offer you advice on all aspects of providing evidence of positive health care effects of your DiGA.
What should be considered in the study?
Evidence of the positive care effect must be provided on the basis of a comparative study according to established scientific standards. The study must show that the use of DiGA is better than non-use in a comparison group aligned with the reality of health care.
The following study concepts are appropriate:
- Clinical or epidemiological studies
- Studies based on methods from other scientific fields such as healthcare research, social research or behavioural research
- Quantitative comparative studies with methods that are adequate for the chosen object of investigation
The studies must be conducted in Germany. In addition, registration registered in a public study registry publication of the complete study results are required.
The inav has extensive methodological expertise in conducting studies with a large variety of evaluation designs. Together, we will develop all documents that need to be attached to the application for admission to the DiGA directory:
- Evaluation concept
- Systematic data analysis
- Statistical analysis plan (SAP)
- Systematic literature search
- Study protocol
How is the price for a DiGA determined?
In the first twelve months after listing in the DiGA directory, the price set by the DiGA manufacturer applies. Thereafter, the price negotiated by the manufacturer and the National Association of Statutory Health Insurance Funds (GKV-Spitzenverband) will be applied.
For DiGAs that were immediately finally listed in the DiGA directory, price negotiations begin four months after the listing. For DiGAs that have been provisionally listed, price negotiations will not begin until the notification of final listing has been received.
Price negotiations are scheduled for a period of five months, during which three to four negotiation meetings are held. If no agreement is achieved in these negotiations, the arbitration board is brought in. It then determines the price after reviewing the documents.
A key factor in determining the price is the strength of the positive health care effect. In addition, factors such as the price of comparable DiGA, the co-payer price and, if applicable, pricing models for the DiGA abroad are taken into consideration.
The inav will guide you in the development of a realistic pricing strategy, while supporting it with significant study data and highlighting the additional benefit of your DiGA.

